-Cytokines
-Carrier proteins
-Recombinant polypeptide
-Recombinant enzymes
-Allergens
-VLPs
-Vaccines
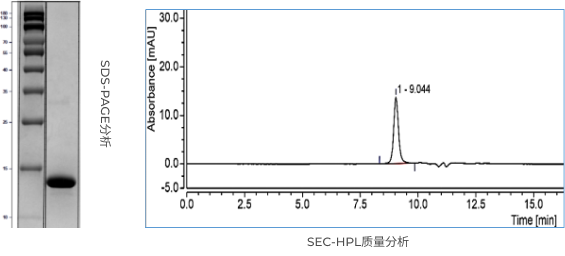
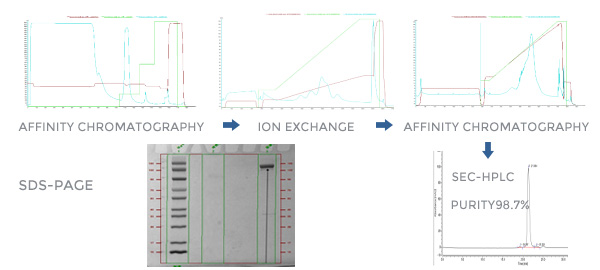
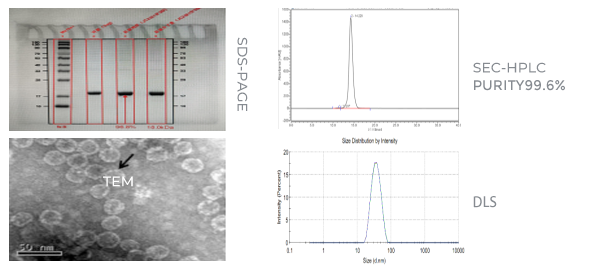
-Mature unlabeled protein process development capabilities to reduce process steps, improve protein purity, and ensure that process impurities and product impurity residues are qualified.
-The localization of critical materials and equipment to save cost, platform-based processes to respond quickly to meet project requirements and shorten process development period.